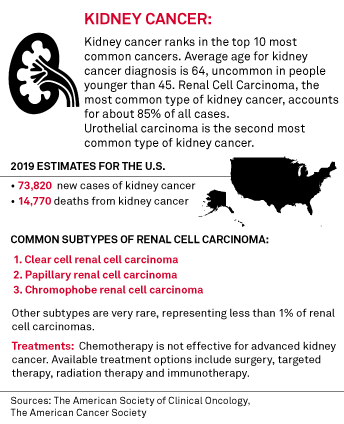
Merck & Co's Keytruda rejected by UK NICE in advanced kidney cancer | S&P Global Market Intelligence

Seagen, Astellas and Merck Announce FDA Acceptance of sBLAs for PADCEV® (enfortumab vedotin-ejfv) with KEYTRUDA® (pembrolizumab) for the First-Line Treatment of Certain Patients With Locally Advanced or Metastatic Urothelial Cancer
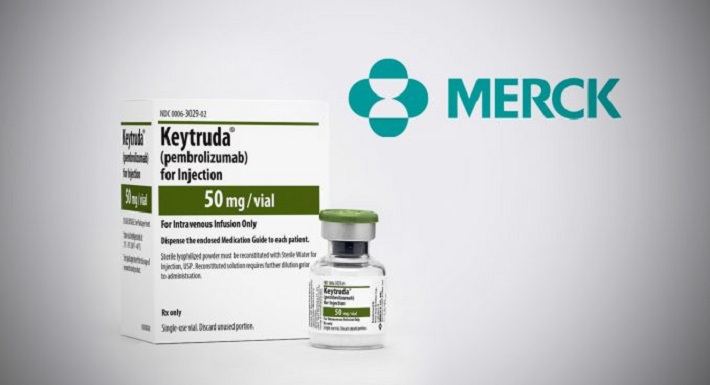
FDA Approves Merck's KEYTRUDA for the Adjuvant Treatment of Patients with Melanoma with Involvement of Lymph Node | World Pharma Today

Merck and Eisai Provide Update on Phase 3 LEAP-010 Trial Evaluating KEYTRUDA® (pembrolizumab) Plus LENVIMA® (lenvatinib) in Patients With Certain Types of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. firmnews.title_view.

Merck's PD-1 Drug Outperforms Ipilimumab for Treatment of Advanced Melanoma - Cancer Research Institute

Keytruda Approved by FDA for Further Potential Treatments of Mesothelioma | Mesothelioma Help Cancer Organization
US FDA approves Merck & Co.'s Keytruda in 2 new head, neck cancer uses | S&P Global Market Intelligence

KEYTRUDA® (pembrolizumab) Showed Statistically Significant Improvement In Disease-Free Survival Versus Placebo As Adjuvant Treatment For Patients With Stage IB-IIIA Non-Small Cell Lung Cancer Regardless Of PD-L1 Expression 2023 - EORTC
FDA Approves Mercks KEYTRUDA® for Patients with Recurrent or Metastatic Head and Neck Cancer | World Pharma Today
Merck Receives Accelerated Approval of KEYTRUDA® (pembrolizumab), the First FDA-Approved Anti-PD-1 Therapy
KEYTRUDA® (pembrolizumab) Plus Padcev® (enfortumab vedotin-ejfv) Reduced Risk of Death by More Than Half Versus Chemotherapy in Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer | PharmiWeb.Jobs United States